Few important questions with answers in stereochemistry for CSIR NET GATE IIT JAM and other National level entrance examinations. We have to briefly in discuss the problem-solving approach that is helpful for your preparation. You can check the preparation strategy in the stereochemistry chapter by solving this type of question. Stereochemistry is the most important chapter for every part of organic chemistry.
Now in advance, organic synthesis stereochemistry is used very widely. For an asymmetric synthesis, different catalyzers used must be mentioned the enantiomer or diastereomer is participating in enantioselective or diastereoselective reactions respectively. That’s very the yield formation of the final product. And approach to the kinetic and thermodynamics product Formation.
Stereochemistry Questions with solution
You can solve those questions manually and practice those topics of stereochemistry questions with the proper Reason. Those questions are from previous years’ CSIR NET June 2011 examinations.
Q.1. The configurations at the two stereocentres in the compound given below are
A. 1R, 4R
B. 1R, 4S
C. 1S, 4R
D. 1S, 4S
Answer: Option A. 1R, 4R
Hint:
The configuration at the two stereocenters in the given compounds below is 1R 4R. we can show so the configuration structures given below…
First of all, we are talking about carbon number 1 The rotation of 1-2-3 clockwise corresponds to group number 4 (Methyl) is below the Plane. Therefore, this chiral carbon is an R configuration.
Now talking about the carbon number 4 the rotation of 1-2-3 is anticlockwise and corresponds to group-4 ( hydrogen) above the plane so the configuration is R.
Note:
If the lowest numbering group is shown above the plane the configuration of that chiral carbons is opposite to that of the direction of rotation.
- That’s why Anticlockwise rotations Show S-configurations when the lowest numbering group is below the plane.
- And clockwise rotations Show R-configuration when the lowest numbering group is below the plane.
- Anticlockwise rotation shows R-configuration when the lowest numbering group is above the plane.
- Clockwise Rotation shows S-configuration when the lowest numbering group is above the plane.
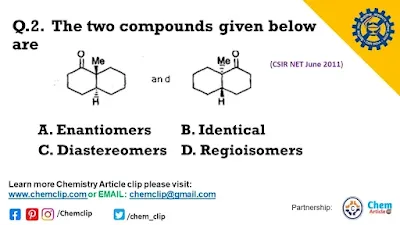
Q.2. The two compounds given below are
A. Enantiomers
B. Identical
C. Diastereomers
D. Regioisomers
Answer: Option B. Identical
Hint:
Given two molecules’ structures are identical. You can check out the configuration at the stereocenters.
The first molecule gives R and S configurations similarly the second compound gives R and S configurations respectively.
Or 3-Dimensional approach:
You can see another approach just like a hand. one hand has a different site – one is front and another is back. That’s why hands on two sides are superimposed on each other. Two identical.
Two hands are identical Or one hand on two different sides, that is called identical.
Two hands are not identical. Or two hands are not the same. The two hands are different. They Give the Mirror image. They are called enantiomers.
Q.3. In the most stable conformation of trans-1-t-butyl-3 methylcyclohexane, the substituents at C-1 and C-3, respectively, are
A. Axial and equatorial
B. Equatorial and equatorial
C. Equatorial and axial
D. Axial and axial
Answer: Option C. Equatorial and axial
Hint:
In the most stable configuration of the trans-1-t-butyl-3-methylcyclohexane, the substituent at C-1 and C-3 are equatorial position and axial position respectively.
According to chare formations of Trans-1-t-butyl-3-methylcyclohexane, you can see the given below structure: carbon numbering-1 is more favorable to equatorial positions and correspond to carbon-3 is their groups favorable to the axial position.
- You can note that the tertiary butyl group always stays in equatorial positions. that is more favorable for their stability.
More Stereochemistry questions and answers
We hope that these types of questions with solutions paper/ article are helpful for you. If you have any queries about those questions and the power portal you can leave a comment in the comment section. Our team solves your problem as soon as possible.
You can follow our Facebook page: @chemclip or Twitter handle: @chem_clip